Background: Patients with COVID-19 have an increased risk of thromboembolic disease, this has been partly attributed to an excessive inflammatory response that is associated with hypercoagulability; patients develop thrombotic complications with rates of 6.4% in non-critically ill and 15 to 31 % in critically ill patients. With this data some clinicians have incorporated thromboprophylaxis with higher dose heparin into the management of this patients, to date there´s no information of the effect of this intervention.
Methods: We conducted a prospective cohort, including consecutive critical and non-critical adults admitted to a referral center in Mexico City, between March 18 and May 19, all with a positive RT-PCR for SARS-CoV 2. Conventional coagulation test results were collected on admission and during hospitalization; use of anticoagulation, and patient outcomes were recorded, all patients had been discharged at the time of the final analysis. Thromboprophylaxis was administered according to institutional recommendations and individual medical criteria, we defined anticoagulant dose according to each medication. We compared the basal characteristics and outcomes in critical and non-critical patients. We evaluated the factors associated with thrombosis, bleeding, and mortality using the Cox Regression Model.
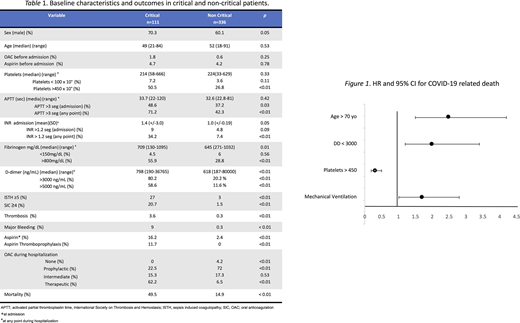
Results: We evaluated 447 consecutive hospitalized patients with COVID-19, median age was 50 years (range,18-91), 62.6 % were male, 111 (24.8%) were critical. At admission 156 patients (34.9%) had D-dimer values above 3000 ng/mL, median fibrinogen was 651 mg/dL (range 130-1095), APTT was prolonged (> 3 seconds) in 179 patients (40%), and INR >1.2 in 26 patients (5.8%), median platelet value 215 X103/uL (range 33-666). Thromboprophylaxis' dosages were prophylactic in 267 (59.7%), intermediate in 75 (16.8%) and therapeutic in 91 (20.4%), 14 patients (3.1%) did not receive any medical thromboprophylaxis and 26 patients (5.8%) received aspirin during hospitalization. According to the International Society on Thrombosis and Hemostasis' criteria (ISTH criteria) 40 patients (8.9%) had overt-DIC, sepsis induced coagulopathy (SIC) was present in 28 patients (6.3%), and high risk for bleeding by IMPROVE score ≥7 points was found in 5 patients (1.1%). Overall thrombotic event (TE) was confirmed in five patients (1.1%), arterial thrombosis events in 0.4%, one stroke and one acute myocardial infarction; radiographically confirmed venous thrombosis in 0.67%, two with pulmonary embolism (PE) and one with deep venous thrombosis (DVT). The TE were more common in critical than in non-critical patients (3.6% vs 0.3%). The number of CT pulmonary angiogram or duplex ultrasounds performed when PE/DVT was suspected was eighteen (4%), eight (47%) non-critically ill and ten (53%) in the ICU; the rate of radiographically positive results was 22.2%. The overall major bleeding rate was 2.5%, of these 91% were in the ICU. Mortality was 23.5% in the cohort. Table 1.
No factors were found to be associated with thrombosis. The factors associated with bleeding were an INR >1.2 (HR 9.0, 95% CI 1.2-67.3, p 0.03), IMPROVE score ≥7 (HR 81.3, 95% CI 11.9-555.6, p < 0.01), and mechanical ventilation (HR 33.1, 95% CI 4.1-262.2, p 0.01). Factors associated with mortality were: age >70 (HR 2.5, 95% CI 1.5-4.2, p <0.01), D-Dimer >3000 ng/mL (HR 2.0,95% CI 1.2-3.4, p <0.01), and mechanical ventilation (HR 1.7, 95% CI 1.01-2.8, p = 0.02). The presence of more than 450x109/L platelets was associated with reduced mortality (HR 0.31 95% CI 0.19-0.50). Figure 1
Discussion: The late outbreak of COVID-19 in Latin America had led to an empiric use of aggressive thromboprophylaxis. Our data shows a low TE rate as compared with other groups, nevertheless we cannot prove a direct impact of the aggressive thromboprophylaxis, firstly because of the low rate or events, and secondly, due to the limitations of an observational study. On the other hand, the incidence of PE/DVT is conditioned by the number of studies performed, yet radiological confirmation has proven difficult due to concerns about virus exposure. Regarding the security of the intervention, major bleeding rates were slightly higher to what has been otherwise reported, but with no bleeding related deaths. The benefit of higher anticoagulant doses most be shown in clinical trials before we can recommend their generalized use in COVID-19 patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal